OUR STORY
Our Story at
Towa Pharmaceutical, the first leading generics company in Japan, internationalized in 2020, established its international hub, Towa International in 2020. Breckenridge is the American affiliate of Towa International.
Towa International engages in research, development, manufacture and marketing of generic and value-added medicines (VAMs) that contribute to improving people’s health.
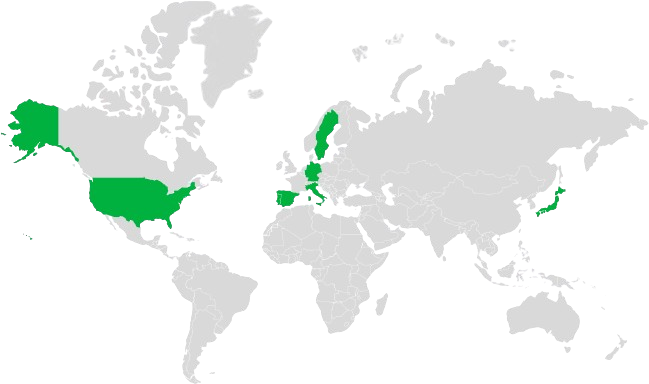
Our aim is to offer Towa’s VAMs in Europe and the US where we distribute, and as well as distributing to more than 60 countries through strategic alliances to reach the greatest number of patients around the world.
We also have a state-of-the-art production plant, a European leader in pellet technology, with a production capacity of over 5.6 billion units per year. Additionally, we boast an R&D center that serves as the international benchmark for Towa Pharmaceutical, our primary shareholder. We are among the top 20 companies globally.
Foundation of Breckenridge Pharmaceutical
Construction of Martorelles (Barcelona) manufacturing and R&D facilities
Creation of B2B Business
Establishment of Pensa Pharma
Approval of our first ANDA, Meloxicam
Start of internationalization phase. Expansion into Italy with the purchase of 20 additional products.
Expansion into AGs and ANDAs to gain entry in the generic drug space
Built pilot plant and expanded R&D capabilities. Enter Portugal through the acquisition of to Life, adding 30 additional product to Pensa’s portfolio.
Approval of our first Paragraph IV (Oxcarbazepine) to gain entry in the generic drug space
Entry in U.S.A with the acquisition of Breckenridge Pharmaceutical. Pensa enters in Sweden.
Acquired by Esteve
Enters Germany
Acquisition of a basket of 25 products expanding beyond solid oral immediate release.
Portfolio Diversification: Focus on drug enabling technology and complex release dosage forms
We marketed our first injectable (Methylergonovine)
Result of Industry Headwinds: Late-Stage deals
Towa Pharma International Holdings is founded.
Acquired by Towa Pharmaceutical
Expands manufacturing facilities.
Over 40 products in pipeline. BPI continues to reinvest, upgrade and build.
Internationalization process. Enters South America and Middle East through licenses.
Shift towards complex products, VAMs and a global focus.
Capabilities expansion
Headquarters in New Jersey
Foundation of Breckenridge Pharmaceutical
Construction of Martorelles (Barcelona) manufacturing and R&D facilities
Creation of B2B Business
Establishment of Pensa Pharma
Approval of our first ANDA, Meloxicam
Start of internationalization phase. Expansion into Italy with the purchase of 20 additional products.
Expansion into AGs and ANDAs to gain entry in the generic drug space
Built pilot plant and expanded R&D capabilities. Enter Portugal through the acquisition of to Life, adding 30 additional product to Pensa’s portfolio.
Approval of our first Paragraph IV (Oxcarbazepine) to gain entry in the generic drug space
Entry in U.S.A with the acquisition of Breckenridge Pharmaceutical. Pensa enters in Sweden.
Acquired by Esteve
Enters Germany
Acquisition of a basket of 25 products expanding beyond solid oral immediate release.
Portfolio Diversification: Focus on drug enabling technology and complex release dosage forms
We marketed our first injectable (Methylergonovine)
Result of Industry Headwinds: Late-Stage deals
Towa Pharma International Holdings is founded.
Acquired by Towa Pharmaceutical
Expands manufacturing facilities.
Over 40 products in pipeline. BPI continues to reinvest, upgrade and build.
Internationalization process. Enters South America and Middle East through licenses.
Shift towards complex products, VAMs and a global focus.
Capabilities expansion
Headquarters in New Jersey